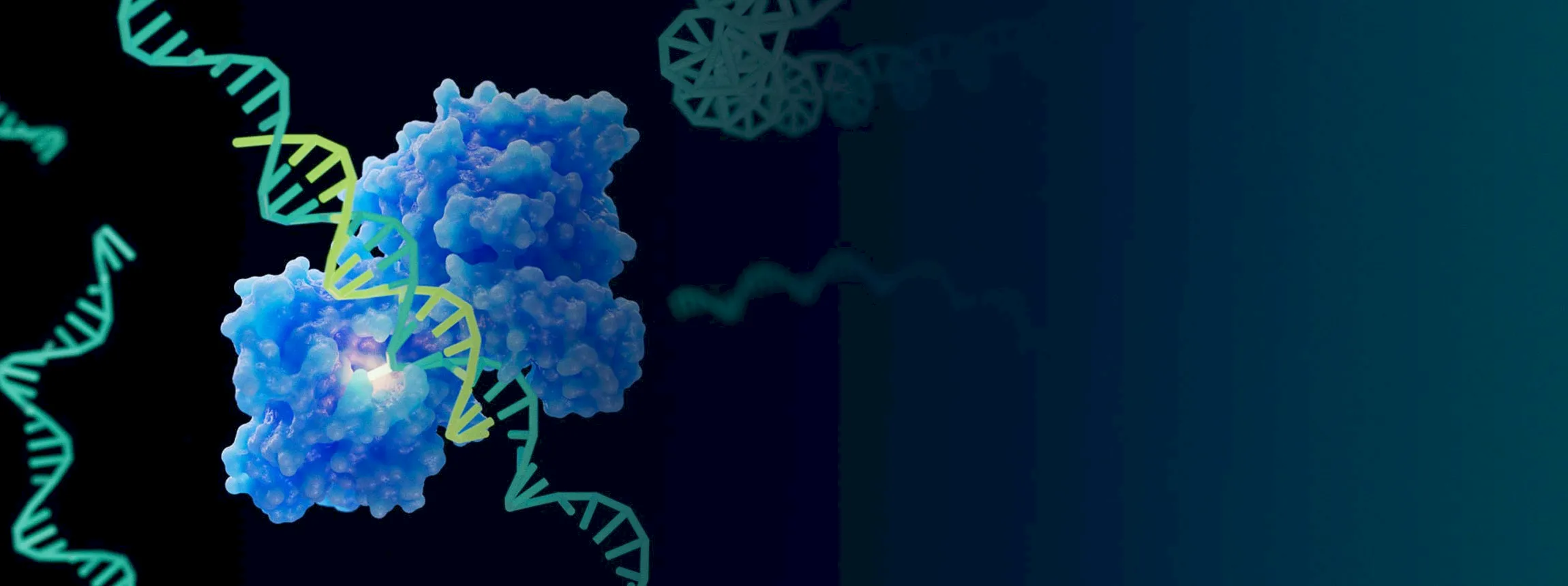
RNA Trans-splicing
The Splice is Right
RNA carries the genetic instructions to make protein. Before the protein can be made, certain unnecessary portions of the RNA are removed. RNA trans-splicing is a technology that hijacks this naturally occurring phenomenon in order to remove the mutated sections of the MECP2 protein RNA and replace it with healthy versions.
RNA trans-splicing is attractive for two main reasons. First, a single RNA trans-splicing therapeutic could treat 97% of all Rett patients. An additional therapeutic would be created to address the remaining 3%. Second, it avoids any possibility of producing too much of the MECP2 protein.
Until recently RNA trans-splicing has been a rather obscure genetic medicine approach without much progress. Academics and biotech companies more recently have embraced this approach.

Omar Abuddayeh, PhD and Jonathan Gootenberg, PhD met while studying and took the unconventional path of joining forces to set up a combined lab. These Genetic Engineers are developing tools to manipulate both DNA and RNA.
Their Rett syndrome program holds the potential to address all MECP2 mutations by “re-wiring” the RNA sequence.

Meet the AbuGoot Dynamic Duo
We sat down with Omar and Jonathan to discuss their journey, their collaborative spirit, and what they aim to accomplish with the funds awarded by RSRT.
Want to learn more about the team? Read a wonderful write-up in the Boston Globe on the pair.

"Like other parents of children with Rett syndrome, Coenraads wishes scientists would hurry up and develop treatments. But as the founder and CEO of the Rett Syndrome Research Trust, she’s in a position to do something about it.”
RSRT featured in a recent Science Magazine article: Buoyed by 'milestone' clinical result, RNA editing is poised to treat diseases.