Using Cerebral Organoids to Develop MECP2-Activating Drugs for Rett Syndrome
Rett syndrome is caused by mutations in the MECP2 gene, which is part of the X chromosome. Cells that lack a working, active MECP2 gene do not develop or function properly and lead to Rett syndrome pathology; however, in female patients, these cells have a healthy copy of the MECP2 gene in reserve, although it has been made inactive as part of the natural process of development known as X-chromosome inactivation. It has long been hypothesized that awakening these silenced healthy genes could reverse many X-linked diseases. Studies have shown that even a small increase in healthy MECP2 protein levels in these cells can reverse symptoms of Rett syndrome. Researchers around the world have been searching for drugs that can activate the healthy MECP2 protein on the inactive X chromosome, but it has been a challenge because there haven’t been effective ways to screen for potential drugs. Animal models lack many aspects of human biology and are not amenable for running many thousands of drug testing experiments.
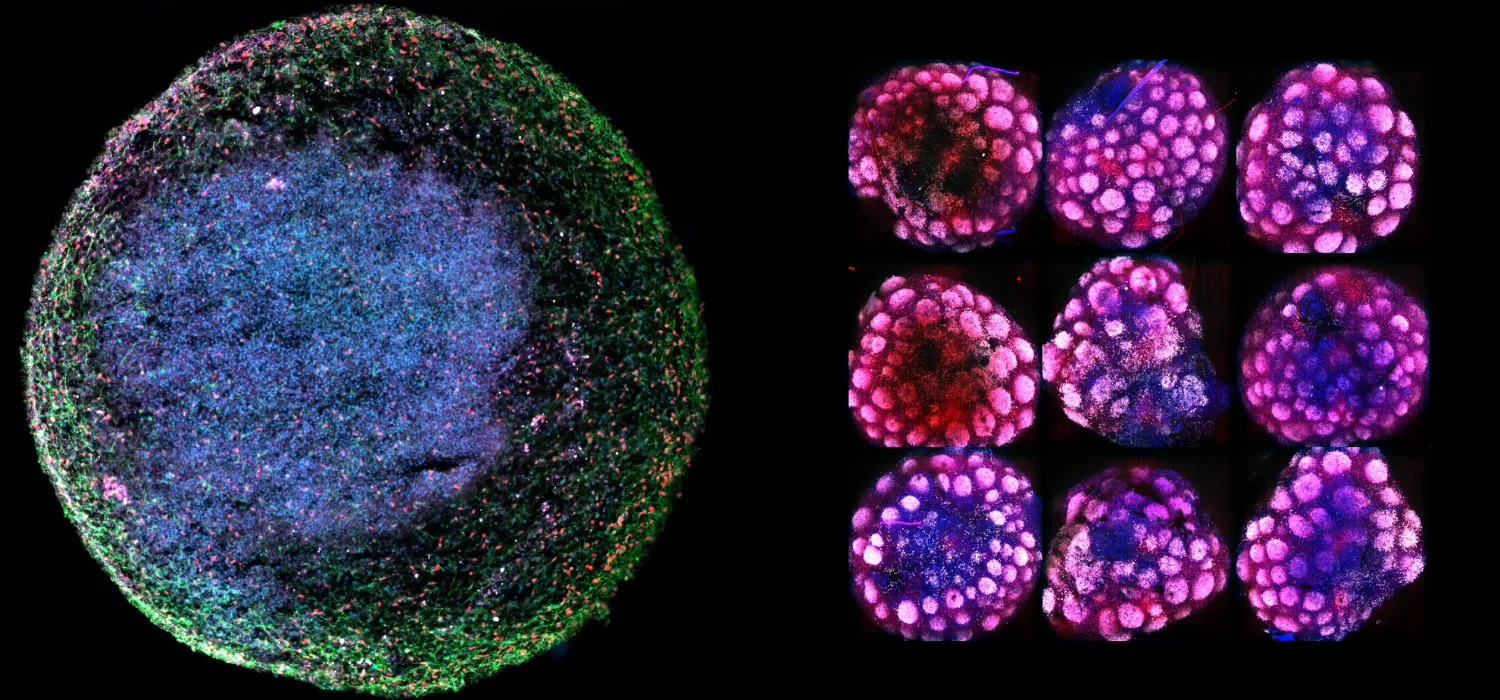
Recently, a new biotechnology breakthrough, called organoids (examples in the banner above), has enabled scientists to build realistic, complex human biological models of various organ types in laboratory culture, paving the way for an innovative approach to studying the microscopic mechanisms of disease and enabling drug discovery at an unprecedented level of biological realism before human clinical trials.
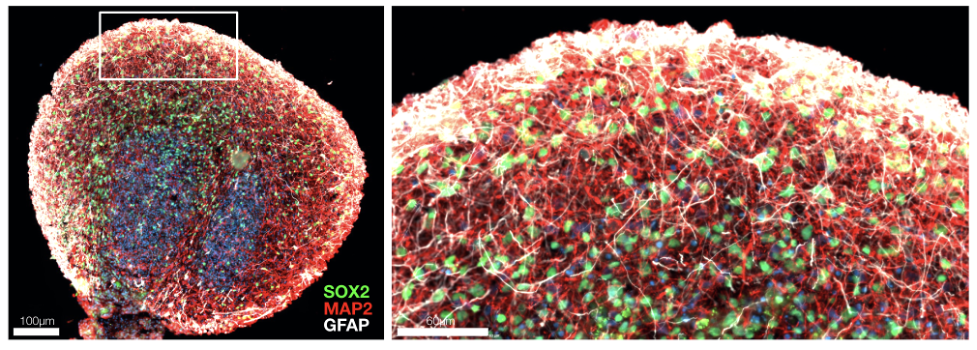
At Herophilus, we have developed a human “cerebral organoid” model of Rett syndrome from patient cell lines we received from RSRT’s biorepository. These tiny brain tissue cultures contain the diversity of cell types of developing human brain tissue and mimic the spatial and cellular architecture of the developing human brain (Figure 1). These organoids are particularly special in that they are derived from Rett patient cells and therefore possess the genetic background of Rett patients, including the MECP2 mutation that causes the disease. These organoids can be used to execute a massively parallel experimental search for drugs that can reverse the disease.
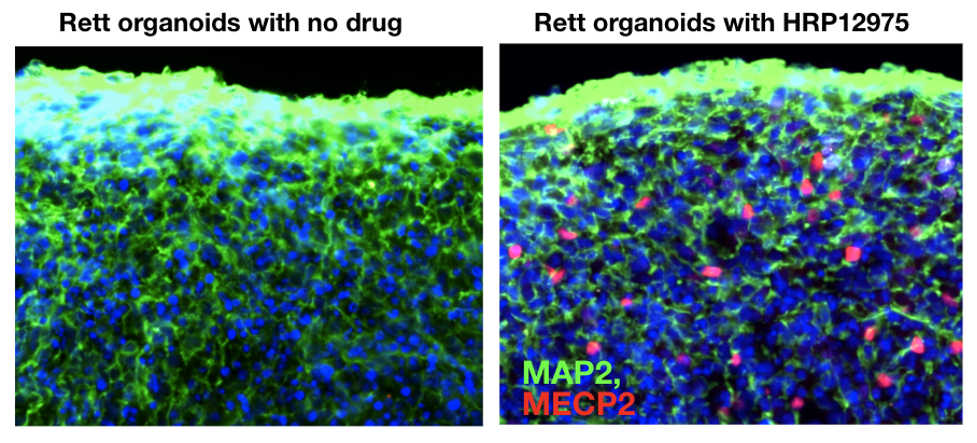
Figure 2. Rett patient cerebral organoids treated for five weeks with HRP-12975 show an increase in MECP2 protein (red) in the nuclei of cells.
At Herophilus, we have developed a robotic platform to grow thousands of cerebral organoids from Rett patients to screen for disease-modifying drugs. Using artifical intelligence-enabled microscopic imaging techniques, we can measure levels of healthy MECP2 protein in neurons and glia, the two major cell types in the human brain. After screening thousands of candidate drugs, we discovered a drug molecule, HRP-12975, that increases the healthy MECP2 protein in up to 20% of the Rett patient cells (Figure 2).
Furthermore, our Rett cerebral organoids have revealed that HRP-12975 enhances the functional structure of the brain tissue by promoting the growth of neuron branches and increasing connections between the neurons, called synapses. This finding is significant because it suggests that HRP-12975 may have truly disease-modifying effects that are observable only in a 3D tissue and cellular model. With funding from RSRT, we are currently evaluating the drug’s safety and tolerability in animal models, a critical step before proceeding to clinical trials in Rett patients.
Our Rett organoid platform embodies a new era in drug discovery, where patient cells can be harnessed in the lab to probe the biological mechanisms underlying disease and to screen for new drugs with an unprecedented level of scale and biological realism. We recognize that none of this groundbreaking research would be possible without the generous contributions from Rett patients and their families, and we are deeply grateful for their ongoing support.


