The Genetic Medicine Revolution is Here!
From 2017-2020 RSRT carried out the first ever strategic research plan, Roadmap to a Cure, with a goal of identifying and advancing genetic medicine approaches that target the root cause of Rett. This resulted in remarkable strides forward. That success was made possible by the families that took action and raised the necessary $33 million, the scientists we fund, and our internal research team that kept its eye on the prize - a cure - and refused to be distracted. Roadmap and the Gene Therapy Consortium that came before it laid the foundation for the current gene therapy clinical trials. Our efforts and results also piqued the interest of biopharmaceutical companies as never before. Remarkably, at the start of 2017 there were zero biopharmaceutical companies pursuing genetic medicines. By the end, in 2020, there were six.
You might ask me—what’s the next phase of the research given all the progress that has been made?
Today I’m excited to announce that RSRT is launching Roadmap to Cures with an ambitious goal of advancing three genetic medicine programs to clinical trials by 2028. The change in title from “a cure” to “cures” reflects our expectations that, in time, there will be multiple cures for Rett. I’m asking you—my fellow Rett families—to join us in this push. Your involvement has never been more important. Let me give you some further background on all of this.
Before my daughter Chelsea was diagnosed with Rett syndrome I had never thought much about how cures for diseases came about. For the past 25 years I’ve thought about little else.
Early on, I learned that cures don’t come about on their own, especially for rare diseases. Foresight is needed to ensure that investments are made to support critical building blocks upon which curative programs can be built: high quality basic science, animal models that recapitulate disease symptoms, multiple genetic medicine programs, patient data, objective ways to measure symptoms in clinical trials, biomarker discovery efforts. With encouraging data in hand, discussions around transitioning genetic medicine programs from academia to biopharma can begin.
Rett is a challenging disease for a number of reasons, which isn't the low-hanging fruit that biopharma gravitates towards. So RSRT’s role is to de-risk as many genetic medicine programs as possible and incentivize biopharma to license them. The effort must be carefully strategized, funded, monitored, and driven every step of the way.
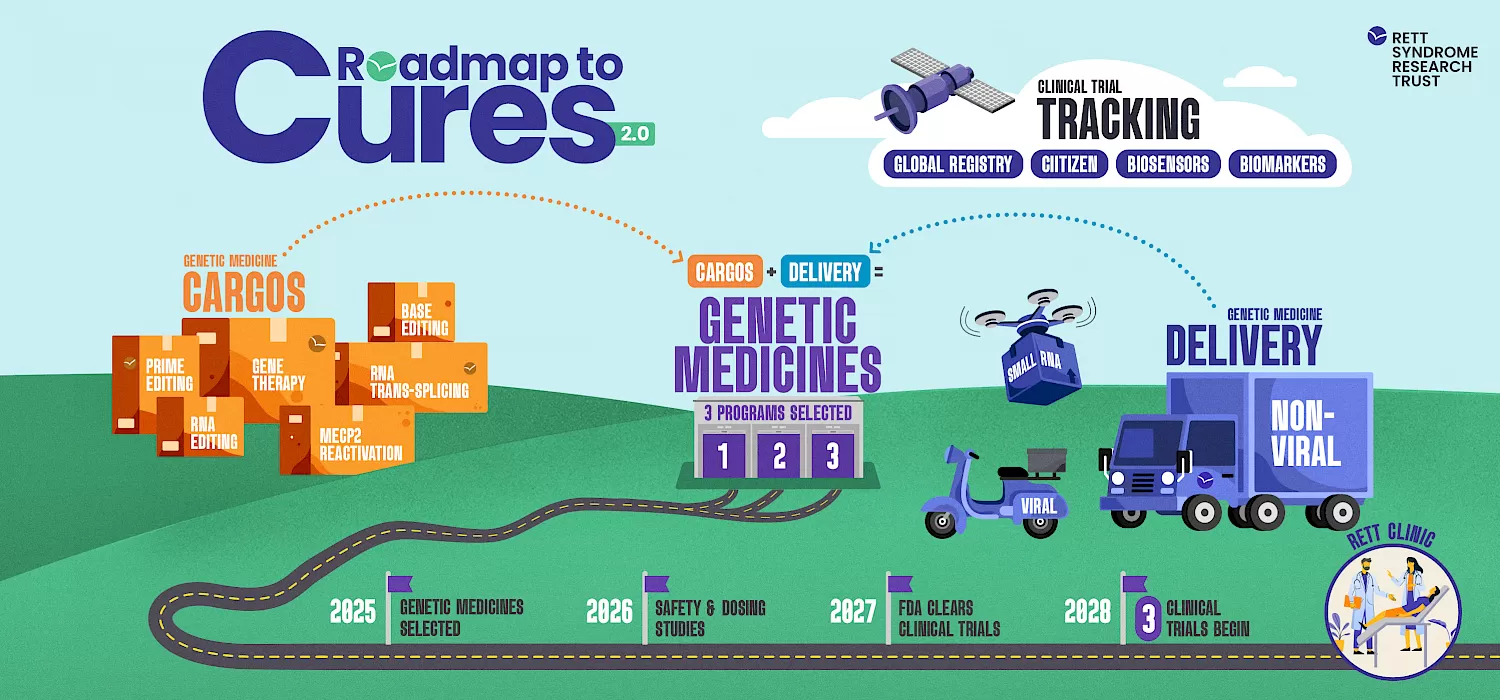
Genetic medicines have two components - a cargo and a delivery technology. The best cargo in the world can’t be of much help if it can’t get to the right cells so both components are equally important. Watch the video below and explore our updated website to learn how Roadmap to Cures aims to advance the development of 3 genetic medicines for Rett.
.
Developing cures is an iterative process and it is unlikely that the first approved genetic medicine will provide a full cure. Our work will not stop when a genetic medicine is approved as next generation genetic medicines will likely provide even more benefit.
We can look to the spinal muscular atrophy (SMA) field which has three approved genetic medicines as an example. Zolgensma is a one and done gene therapy; Spinraza is dosed three times a year; and Evrysdi is an RNA-based drug infused three times a year in combination with a daily medicine. Some patients have received these medicines in combination. SMA is a neurodegenerative disease so treating early is key. In some patients these drugs have provided dramatic results. However, the SMA work continues with other genetic medicines and drugs in development including next generation gene therapies.
I want to take a moment and address a question that most of us have asked ourselves: What is a cure and what will it look like for my loved one? In its purest form a cure means “like it never even happened”. Will a “like it never even happened” scenario be reserved for in utero administration, or for newborns or toddlers or can it be achieved in children as well? What about adults? Truth is, no one knows. Current and future clinical trials will provide answers.
Fortunately, there are lots of possibilities beyond the “like it never even happened” scenario. My daughter is 27 years old and I don’t expect a cure for her. But I am optimistic that many of her symptoms could dramatically improve. I think most of us would welcome dramatic improvements over the current status quo.
The best chance we have of providing effective all out cures or the closest we can get to cures for all of our children is to pursue as many shots on goal as our resources allow. It won’t come as a surprise that none of this is easy or cheap. To execute on Roadmap to Cures we need to raise a minimum of $10 million a year for the next four years. Please help us to help your loved one.


