Stepping Out of The Dark Ages and Into The Data
Clinical development for neurological disorders like Rett syndrome is in desperate need of modernization. So how do we fix it?

I was recently inspired by a post entitled You're so Vain, by William Hirst at Impatient Health, around the shortcomings of defining success in pharma when focused on the wrong things. It resonated with my frustrations about how Rett symptoms are measured in clinical trials and clarified for me just how important our biosensor development program is. Thanks for the inspiration, Will.
“You can’t manage what you
can't measure.”– Peter Drucker, Management Education Pioneer
This statement is profoundly applicable to achieving cures for Rett syndrome. Clinical development, or the art and science of drug making, is stuck in the dark ages when it comes to measuring symptoms in neurological disorders like Rett. Though we have the technology to monitor our day-to-day health through steps, sleep quality, resting heart rate, and many others through our smartphones, watches, and other devices, for Rett we still test medicines in clinical trials through human observations, assumptions, and guestimates, without directly measuring a single symptom in the patient.
At RSRT, we recognize that if we're measuring the wrong things, we're managing the wrong things, too. And if it turns out we’re not measuring anything, then what exactly are we managing? This is why our biosensor development program is a game-changer. Technology is advancing at record pace, now enabling us to accurately measure Rett symptoms in patients directly. And we’re taking this capability to the front lines of drug development to get cures to our loved ones faster.
Rating the Rating Scales
Questionnaires completed by parents and clinicians who must observe and interpret an individual’s Rett symptoms at specified times during a trial are the standard way therapeutics are currently evaluated and approved. The problem is, rating scales measure what appears to be rather than what actually is. They are inherently subject to biases. For example, were the past few mornings particularly good or bad and therefore disproportionately influencing a caregivers memory of symptoms over the past month? If there is symptom improvement, is it real or is it wishful thinking or a heightened awareness of positive or negative events? Or what if the symptom under study isn’t present while they’re being observed by the doctor, or what if it is present but it isn’t the same as when they’re at home during the other 700+ hours between study visits?
These flaws are one reason why trials using rating scales to determine if a drug is working need so many patients and take so long to show change. When you have a lot of variability around a small number of imprecise scores it makes it very difficult to see a signal above the noise, especially if that drug has small effects.
The Alternative: Real-Time Monitoring
Biosensors have the ability to eliminate many of these uncertainties. Firstly, they measure symptoms directly in the patient and reflect the true symptom burden. Secondly, they monitor symptoms continuously. This means you have information on symptoms day and night, over weeks, or even months at a time and therefore a better understanding of how symptoms are impacting an individual.
Additionally, biosensors automatically detect the symptom event, like a heartbeat, a breath, an oxygen saturation, a step, in the same exact way every time. This is a huge advantage over rating scales because you remove the biases in determining if there was a symptom event or not, and you can begin to actually count and quantify events into frequencies and durations with precision. We can even measure symptoms like heart rate or oxygen saturation that can’t be observed or rated on a scale at all.
In the fight against Rett syndrome, traditional rating scales that take fuzzy snapshots of symptoms from someone else’s perspective don’t offer the necessary insights to determine if a therapeutic is leading to real change for a patient. Biosensors, on the other hand, give us a continuous stream of meaningful data that when used over longer periods of time can show us much deeper insights about change, including precisely at what pace that change occurred. Our goal for the biosensor development program is to provide options to confidently track real-time symptom changes in patients, ensuring that we answer the critical question: are symptoms actually improving or not?
VIBRANT: Moving Beyond Rating Scales
In our VIBRANT study, we are comparing heart rate, breathing patterns, sleep, and oxygen saturation from biosensors to the gold standard (eg. sleep studies) that doctors would rely on in the clinic to learn about their accuracy and reliability. The outcomes of the study will elevate the field far beyond surface-level rating scales, paving the way instead to vetted objective symptom measurement options can that provide a detailed and dynamic picture of a patient's symptoms over time.
To drive drug development to meaningful therapeutics we need meaningful measurements. We need direct and objective measurements of the true impact of a therapeutic on symptoms in a quantitative unbiased way. Biosensors offer a solution by providing precision about how a drug is working, requiring shorter time periods and fewer patients, ultimately accelerating the development process.
The Bottom Line
It is daunting to change the current paradigm of drug development’s reliance on rating scales, but biosensors hold the key to bringing the future of curing Rett syndrome closer. We now have the tools to measure real change in Rett patient symptoms, and by de-risking and bringing these tools to drug development through VIBRANT we are moving the goal posts up. We all know appearances can be deceiving. It’s time to get out of the dark ages and let biosensors objectively measure what truly matters.
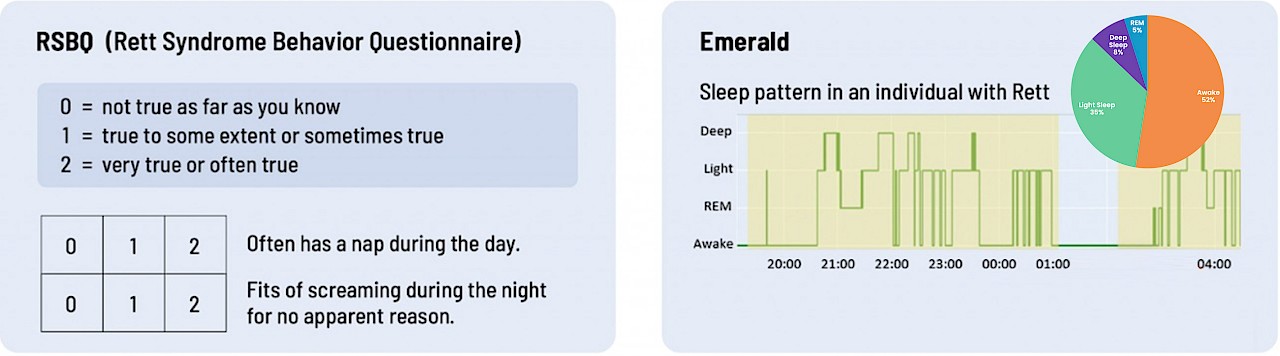
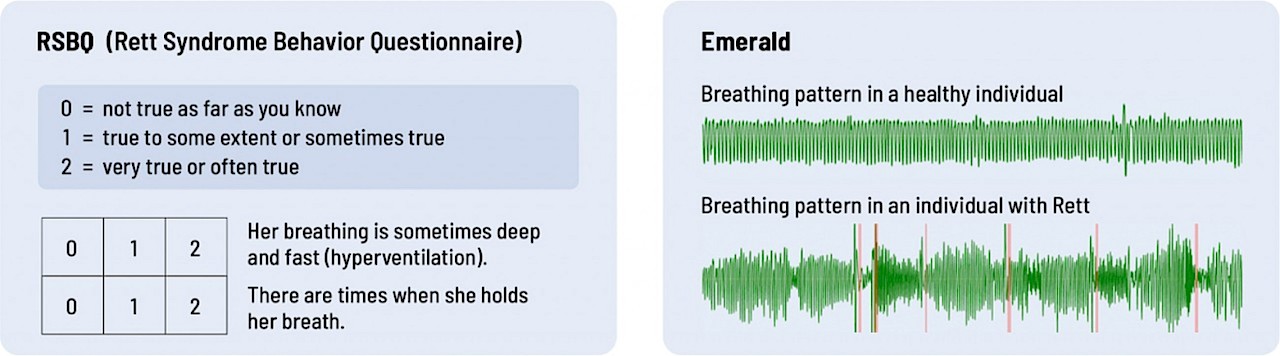
Check out the examples below: Which do you think provides more accurate data on breathing and sleep?
.
Breathing
Sleep