Breaking Barriers: New Pathways Into the Brain
This Cutting-Edge Delivery Tech Could Transform How We Treat Rett.

RSRT is teaming up with Apertura Gene Therapy to bring TfR1 CapX, a groundbreaking new delivery technology, into our genetic medicine programs. TfR1 CapX’s ability to non-invasively target the brain at low doses could be a game changer for treating Rett syndrome and other neurological disorders.
A Big Development in Delivery
You’ve all heard the saying that in real estate, it’s all about “location, location, location.” But when it comes to developing genetic medicines for Rett and other devastating conditions, success rides on “delivery, delivery, delivery”— how the treatment actually makes its way to the target, in our case…the brain.
We’re constantly looking for promising new delivery approaches, which is why we’re excited to tell you about our new partnership with Apertura Gene Therapy. This biotech company, founded on the research of Ben Deverman at the Broad Institute in Boston, has developed a novel delivery technology that RSRT has licensed.The license agreement gives us the ability to use the technology for our internal genetic medicine programs, as well as sublicense it to companies and academic institutions.
To understand just how inspiring this development is, let’s take a closer look at the role of delivery in genetic medicine and what Apertura is doing to really push what’s possible in exploring better and safer delivery technologies.

Dave Greenwald, PhD | Executive Chairman of the Board, Apertura Gene Therapy
Why Delivery Matters
The whole idea behind genetic medicine is to get a cargo (whether it’s a gene or an editor that can fix a specific mutation or mutations) into as many cells as possible. Getting the right cargo to the right place is super important, because the more cells fixed, the more improvement a person with Rett is expected to experience.
So, what’s the best way to do this? Delivery technologies fall into two main categories: viral and non-viral. The viral delivery approach is just like it sounds: it uses a harmless virus as a tiny Trojan Horse, harnessing the vector’s natural ability to ‘sneak’ its cargo into host cells. The non-viral approaches encompass pretty much everything else. These other technologies have their advantages, but remain a work in progress.

Overcoming the Barriers
For now, viral vectors, and specifically, the adeno-associated virus serotype 9 (AAV9), are the gold standard for delivering genetic medicines to the brain. But they have their work cut out for them. Our brains are protected by the blood-brain barrier (BBB), a protective filter of tightly packed, specialized cells designed to keep harmful substances (like viruses) out of the brain while letting much needed nutrients (like oxygen and iron) in.
Current Rett gene therapy clinical trials get around the BBB by injecting medicines into the cerebral spinal fluid that bathes the brain and spinal cord. Still, some parts of the brain remain inaccessible via these routes of administration.

For substances that would be shut out of the brain because of the BBB, the brain has an array of receptors that regulate how substances get in and out. One of them, the transferrin receptor (TfR1), operates like a ferry, shuttling iron from the blood into the brain.
This is where Apertura comes in. Through its research into viral delivery methods, the company uses TfR1 to move its capsid from the blood into the brain. It does this through changes on the capsid’s protein shell “suitcase” that surrounds the virus cargo, enabling it to bind to the transferrin receptor. This means the genetic medicine cargo can now hitch a ride through the BBB and into the brain, where it can begin its important work fixing cells.
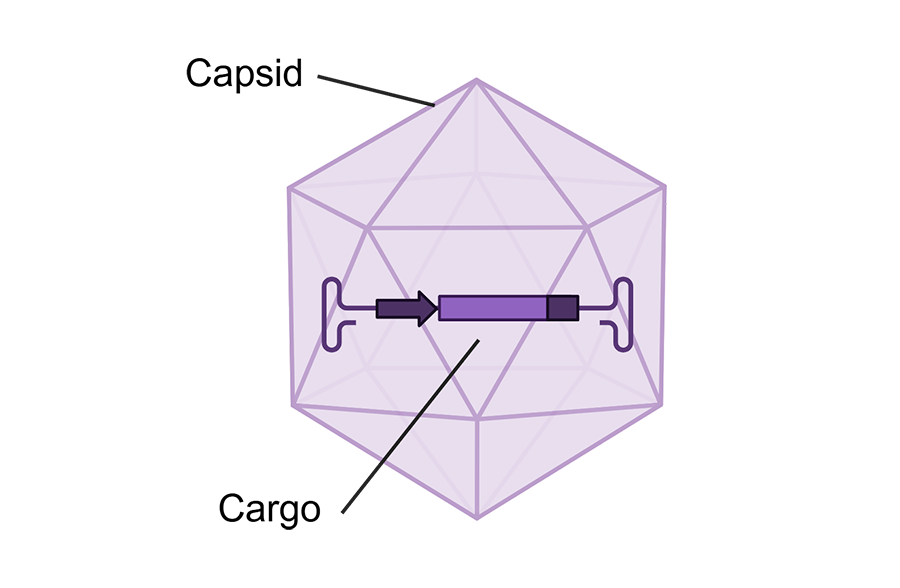
The Rap on TfR1 CapX
What makes CapX such an exciting technology to the Rett research community is its unique advantages over other gene therapy delivery approaches.
Firstly, CapX can be delivered into the bloodstream with a simple injection, just like getting IV fluids. It doesn’t require a lumbar puncture or the neurosurgery required for delivery to the brain’s ventricles.
Secondly, because CapX is injected into the blood it can access the brain’s extensive network of blood vessels allowing for efficient and diffuse distribution of its cargo.
Thirdly, since the capsid gets into the brain more efficiently, lower doses are likely to be needed. Much lower. For example, the expected dose using CapX is 50 times less than Zolgensma, the approved gene therapy on the market for spinal muscular atrophy (SMA). Lower doses means potential fewer concerns about toxicity and cheaper manufacturing costs.
This capsid approach has yet to be tried in humans. At present, CapX is being developed in the preclinical stage for two serious brain disorders: tuberous sclerosis complex 1, a form of genetic epilepsy, and prion disease. This means that before any Rett programs get into the clinic, there will be essential human data using this capsid.


