RSRT Awards $530,000 to Neurolixis for Clinical Development of NLX-101

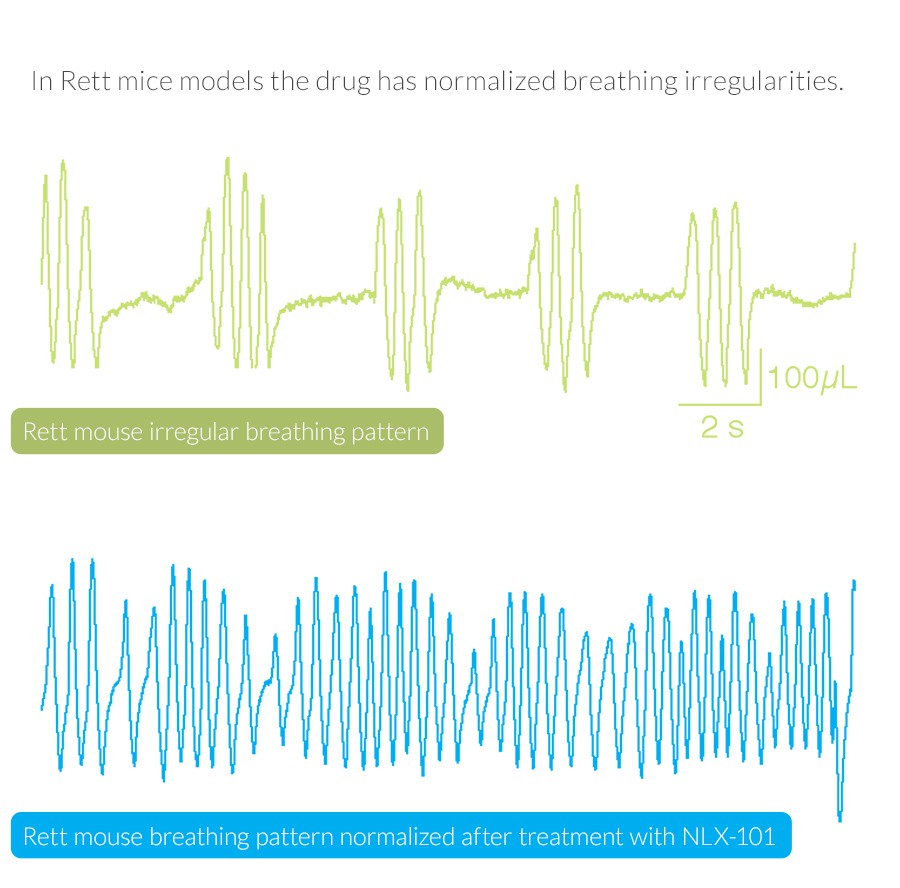
RSRT recently awarded $530,000 to Neurolixis, a small biotech firm in southern California that is developing the drug, NLX-101, to treat breathing abnormalities in people affected by Rett Syndrome. The drug targets a specific serotonin receptor (5-HT1A) located in regions of the brain that affect respiration, mood and cognition. It’s possible that, beyond breathing, the drug may also improve other core symptoms such as anxiety and movement disorders.
Neurolixis has already obtained Orphan Drug status for NLX-101 in both the US and in Europe. This designation provides the company with certain financial incentives as part of the Orphan Drug Act.
Previous RSRT funding to Neurolixis focused on studies to determine dosage levels for human studies. The next step is for Neurolixis to file an Investigational New Drug (IND) application with the FDA before clinical testing of the drug can begin.
The current award will be used to manufacture and characterize clinical supplies of NLX-101, and to prepare regulatory documents for submission to the FDA. The goal is to have the IND submitted to the FDA within a year. Once the IND is open, Neurolixis will test the safety, tolerability and pharmacokinetics (the time course of the drug’s absorption, bioavailability, distribution, metabolism and excretion) in healthy volunteers and in people with Rett.
By supporting this program, RSRT will help Neurolixis “de-risk” the project and make it more attractive to investors, who can support the next stage of development and expedite the process.