Mutations Fighting Mutations

In a new study, mouse #895, also known as Henry the 8th, outlived most other models of Rett Syndrome by years. Another mouse, Fred Astaire, was slim and agile, with a good neurological score. Bruiser was brawny with good muscle tone.
All of these mice were missing MECP2, the gene responsible for Rett Syndrome. In people, MECP2 can be mutated, causing a rare genetic neurological disorder that can lead to severe impairments in the ability to speak, walk, eat and breathe. But these Rett model mice also had something extra: mutations in other genes that improved their symptoms, presumably by compensating for some of what goes wrong when MECP2 doesn’t function normally.
The three mice starred in a large genetic screening project in the lab of molecular geneticist Monica J. Justice, head of the program in genetics and genome biology at The Hospital for Sick Children, in Toronto, Canada. In a new paper, the team found that proteins affected by protective mutations in mice clustered into several biological pathways. The data may lead to greater understanding of disease pathology and to new treatment strategies.
“We are trying to figure out if two wrongs make a right,” Justice says. “Our basic question when we first started this project was: Is there a second locus in the genome that, when altered or mutated, could change the outcome of the phenotype caused by an MECP2 mutation?”
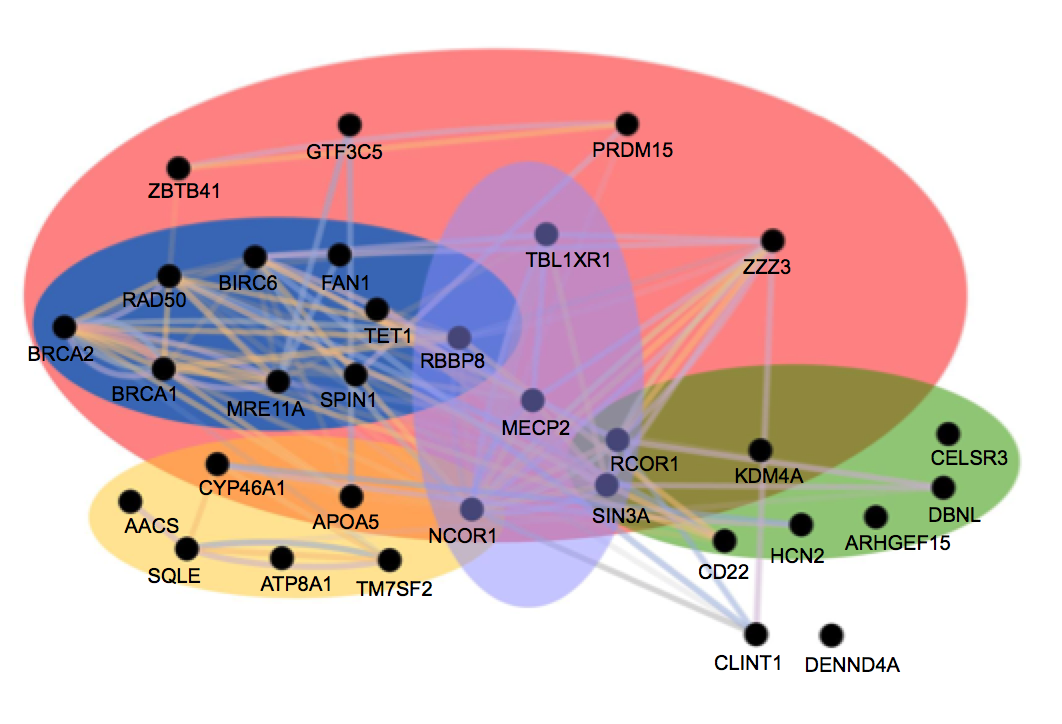
The answer is yes, at least in these mice, according to the study published in the April 2020 issue of the journal Genome Research. The genes fall into three main functional categories.
In the most surprising finding, the largest group of protective mutations involved the DNA damage response. For example, the mouse Bruiser’s phenotype arose from the mutated DNA damage response gene Tet1, relieving typical symptoms of limb clasping, poor muscle tone and lack of movement.
In many neurons, double stranded breaks allow essential genes to be transcribed quickly, Justice says. “We know a lot about this pathway in dividing cells, but we don’t know exactly what is happening in neurons.”
Other researchers have reported increased DNA damage in developing neurons with MECP2 mutations, but in this study DNA damage repair proteins were elevated in mature neurons that do not divide.
“The findings suggest that how neurons repair their DNA damage is important,” Justice says. “Double-stranded breaks can be repaired precisely, or can be repaired more quickly by healing the DNA in a less precise way. Surprisingly, the data from the screen suggest that repairing the DNA quickly rather than precisely is important for Rett cells.”
“It’s unexpected, so we don’t yet understand what we could do that could be therapeutic,” Justice says. “One of the things my lab is doing is to find out why this is important and if there is anything we can do.” For example, existing cancer drugs target DNA repair mechanisms, she says, but they are designed to kill cells rather than restore function, as would be desired in a Rett Syndrome therapy.
In other findings, the study added more evidence for two other categories of genes that have been shown to ameliorate symptoms in mouse models of Rett Syndrome. Six of the genes clustered in the cholesterol metabolism pathway. Henry the 8th is an example of a Rett mouse model that thrived with a single mutated gene in the lipid pathway.
That gene was described in an earlier pilot study by Justice and her co-authors. In the 2013 paper, the team also reported that statins could mimic the protective genetic effect in mice with MECP2 mutation. Other researchers also found cholesterol synthesis was disturbed, but reported that a statin did not have the same effect in Rett model mice with a different genetic background. Results of a small open label clinical trial testing the efficacy and safety of statins in girls with Rett have not been published but were inconclusive, says Monica Coenraads, executive director of the Rett Syndrome Research Trust (RSRT), which funded both Justice studies and the clinical trial.
The third category of mitigating genetic mutations cluster around neuronal signaling or synaptic activity, which other researchers have implicated previously. The mouse Fred Astaire had an additional mutation in this category. Two other categories are transcriptional repression and regulation of DNA activity, which are gene expression pathways that involve MECP2.
In all categories, some symptoms, such as overall health, longevity and limb-clasping behavior, improved in mice with different mutations in some genes. “It’s not like Adrian Bird’s study, where he reintroduced the MECP2 gene, and mice got so much better,” Justice says, referring to a 2007 study by the University of Edinburgh, Scotland, team that first suggested the possibility that Rett Syndrome could be reversed in girls after symptoms had emerged.
“The other unexpected thing we learned from the project was that some mice had two mutations that occurred in the different gene clusters, and those two mutations improved symptoms better than one alone,” Justice says.
It’s too early to make therapeutic predictions on the findings of the mouse genetic screening. In the absence of a therapeutic that replaces the function of MECP2 itself, Justice thinks treatments to modify other pathological pathways may require combination therapies.
In some respects, the notion of inducing more mutations to improve symptoms from another mutation seems counterintuitive. But MECP2 binds extensively to DNA, likely modulating multiple biological pathways.
The technique used in this study, genetic screening for modifier genes, is usually conducted in short-lived fruit flies or round worms to uncover pathways for gene functions, Justice says. But MECP2 is not found in fruit flies or worms. Yet, because the screen is completely unbiased, “animals tell us what’s important,” she says. "Often, we have been totally surprised, then after generating more data, the reason why symptoms improve makes complete sense."
So, she and her colleagues applied modern technology and adapted statistical techniques from human genome-wide studies to minimize the number of mice needed for the study. Although Rett Syndrome primarily affects girls, the researchers used male mice for the screen, because, in contrast to people and female mice, males show symptoms early and consistently.
The genetic screen identified 106 founder mice missing MECP2 but with other mutations that showed improvement in symptoms and longevity. The researchers identified the genes through massively parallel genetic sequencing and verified phenotypes through cross breeding and association analysis.
“The goal of this paper was to report everything we have found up until now,” Justice says. Plenty of work remains, including sequencing a few remaining founders and confirming other modifiers of Rett-like symptoms in other mouse founder lines.
Her lab will be following up on the DNA damage pathway as soon as the coronavirus pandemic restrictions allow, especially genes responsible for repairing double-stranded breaks.


