DAYBUE (trofinetide): Key Facts for Parents

For a disorder as devastating as Rett syndrome, the announcement of an FDA-approved treatment is important. While the new drug DAYBUE (trofinetide/NNZ-2566) is not a cure for Rett syndrome, any reliable improvements in the debilitating symptoms of the disorder are most welcome.
Since the FDA approval of DAYBUE, we have received many inquiries from parents asking for our help in understanding the results of the LavenderTM study to make an informed decision about whether to try the medication for their child. Many questions have come from families outside the US who are deeply concerned that their child may not have access to DAYBUE for years to come, if at all.
Families are struggling to understand the statistical concepts and the other information shared by Acadia Pharmaceuticals. In our capacity as an organization dedicated to curing Rett syndrome, here are facts from the publicly available information, including results of the LavenderTM trial and the official prescribing information for DAYBUE, that in our opinion are important to discuss and consider with your physician as you make your own risk: benefit assessment about whether or not to try DAYBUE.
Download a PDF of this document to share with your physician.
We hope families will find this summary helpful.
Key Summary Facts of LavenderTM and LILAC-1 Trials
- 61% of patients taking DAYBUE did not improve
- 13% of patients were rated as “much improved”
- No data are provided regarding which specific symptoms improve
- 85% of patients treated with DAYBUE had diarrhea and 29% had vomiting
- In the study where everyone received DAYBUE, 46% of patients withdrew before completing the study
Trofinetide Development Timeline
Trofinetide is the chemical name for DAYBUE. Based upon efficacy in a rat model for traumatic brain injury (TBI), a clinical trial with trofinetide for treatment of TBI was initiated in 2008. In that study, trofinetide was administered intravenously. It did not demonstrate efficacy for treating TBI.
Trofinetide was then re-formulated as an oral solution and tested in individuals with concussion, Rett syndrome and Fragile X syndrome. DAYBUE’s official prescribing information states that its “mechanism of action” is unknown. This means that how DAYBUE produces an effect in Rett syndrome is not known. Although there is a publication reporting the efficacy of trofinetide in a Fragile X mouse model, there are currently no publications evaluating the efficacy of trofinetide in any animal model of Rett.
DAYBUE has been previously tested in a number of clinical trials in individuals with Rett syndrome. A clinical trial in adults (initiated in 2012) and a clinical trial in pediatrics (initiated in 2016) assessed its safety and tolerability.
The FDA approval of DAYBUE is based on the LavenderTM clinical trial, which began in 2019. In the prescribing information the trial is referred to as Study 1.
As shown in the table above, the LavenderTM trial had two primary clinical outcome assessments: RSBQ and CGI-I. A clinical outcome assessment aims to reflect how a patient feels, functions, or survives. Both the RSBQ and CGI-I are scales that require caregivers and clinicians to interpret how their child and patient are doing and are therefore subjective by nature.
What is the RSBQ?
The Rett Syndrome Behavioral Questionnaire (RSBQ) is a 45-item survey that assesses behavioral and emotional characteristics of Rett.
Here are some examples of symptom statements included in the RSBQ.
- Her breathing is sometimes deep and fast (hyperventilation).
- Spells of screaming for no apparent reason during the day.
- There are certain days/periods where she performs much worse than usual.
- Has frequent naps during the day.
- Expressionless face.
- Bright wide-open eyes.
- There are times when she is irritable for no apparent reason.
- Rocks self when hands are prevented from moving.
In the LavenderTM study the RSBQ was used to assess the severity of Rett symptoms at different time points. Caregivers rated each item with a 0, 1 or 2 based upon the following criteria:
- 0 = the item is not true for an individual
- 1 = the item is somewhat or sometimes true in the individual
- 2 = the item is often or very true in the individual
Since there were 45 items, each scored from 0 to 2, the lowest possible score is 0, and the highest possible score is 90. The higher the score the more severe the symptom rating.
RSBQ Results
In LavenderTM study, 94 patients were assigned to receive placebo and 93 patients were assigned to receive DAYBUE. The table below is from DAYBUE’s prescribing information and reports the RSBQ results.
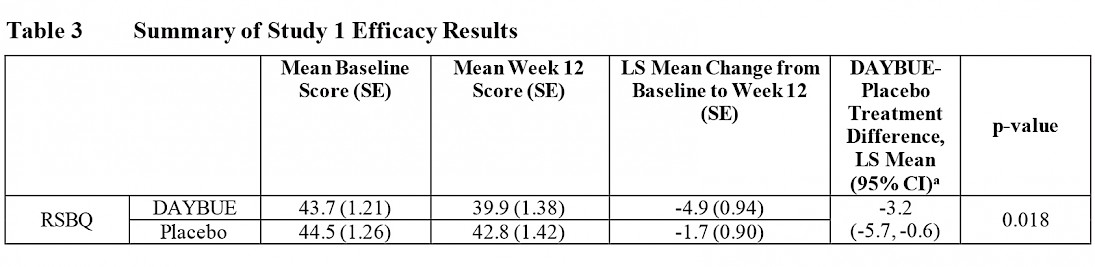
This table shows that the RSBQ average baseline score (at the start of the study) was 43.7 for those taking DAYBUE and 44.5 for those on placebo, meaning that severity scores on the RSBQ were similar for both groups before starting treatment. At Week 12 (which was the end of the study), the average score for the group of patients receiving DAYBUE improved by 4.9 points, while the placebo group improved by 1.7 points. The difference between an improvement of 4.9 and 1.7 points is statistically significant.
Below, we present the data in two ways: one way emphasizes the statistical significance of the difference between DAYBUE and the placebo, while the second way focuses on the size of the difference.
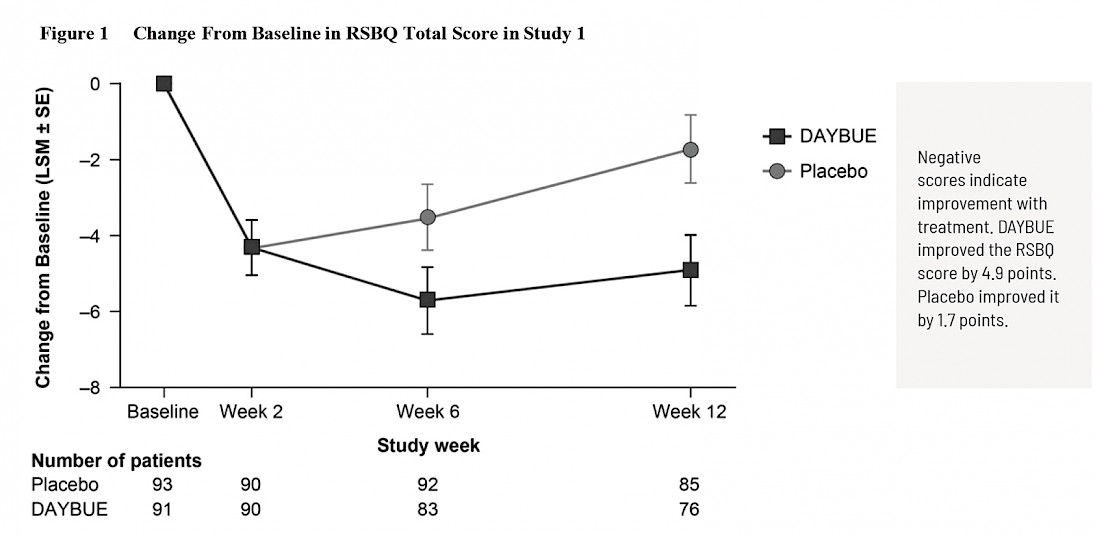
The first graph is taken from DAYBUE’s prescribing information and shows a “close up” of the change observed over 8 points of the 90-point scale.
The second graph shows the same week 12 data, but this time instead of change, total scores at each visit are shown on the full scale for the RSBQ.
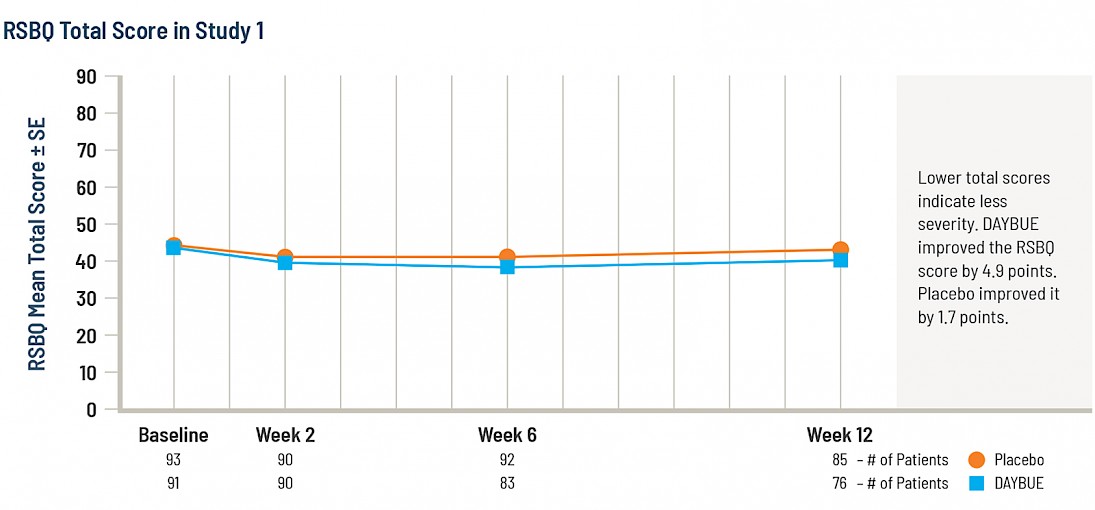
It is not possible to conclude whether a 3.2 point difference on this scale is clinically significant as there are no publications that define what constitutes a meaningful change in the RSBQ score.
What is the CGI-I?
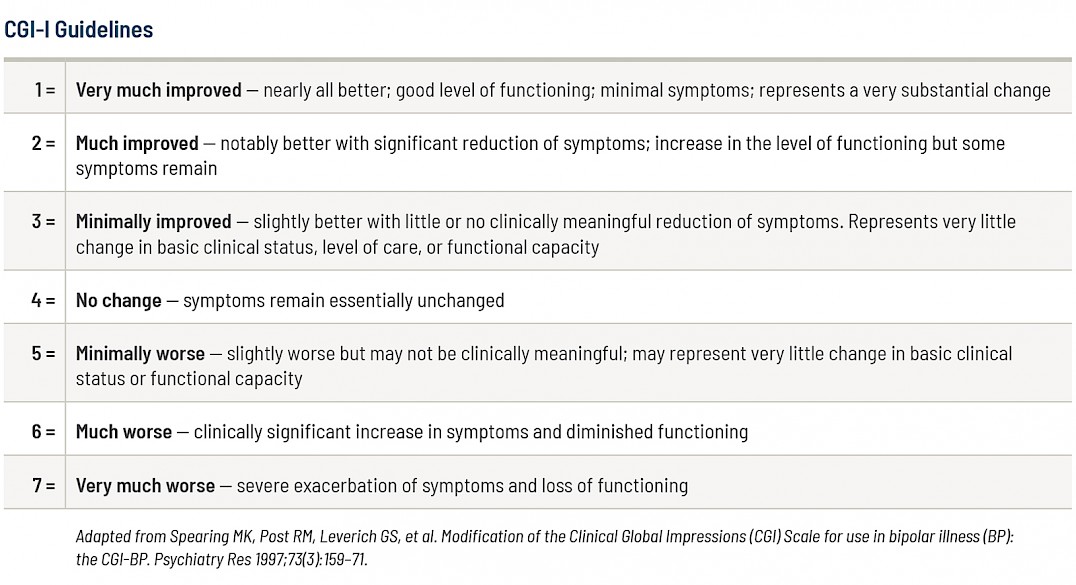
The Clinical Global Impression – Improvement scale (CGI-I) is a seven-point scale where the clinicians assess how much the patient’s illness has improved or worsened over the 12 weeks of treatment. The scores range from 1 to 7:
1 - Very much improved
2 - Much improved
3 - Minimally improved
4 - No change
5 - Minimally worse
6 - Much worse
7 - Very much worse
Click here to see the adaption of the scale for Rett that was used in the study.
CGI-I Results
The table below presents the CGI-I efficacy results in the LavenderTM study as excerpted from the DAYBUE prescribing information.
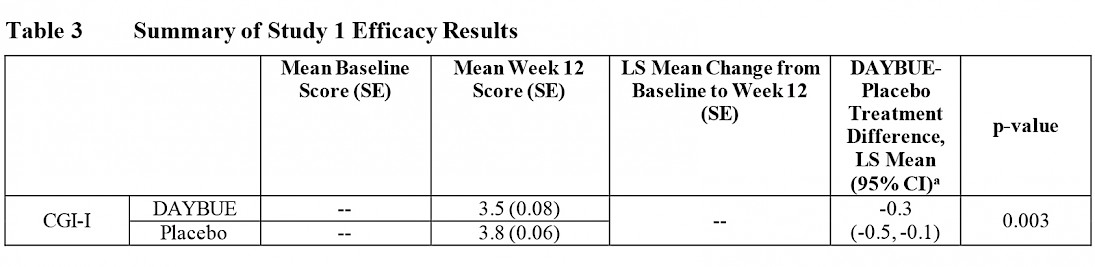
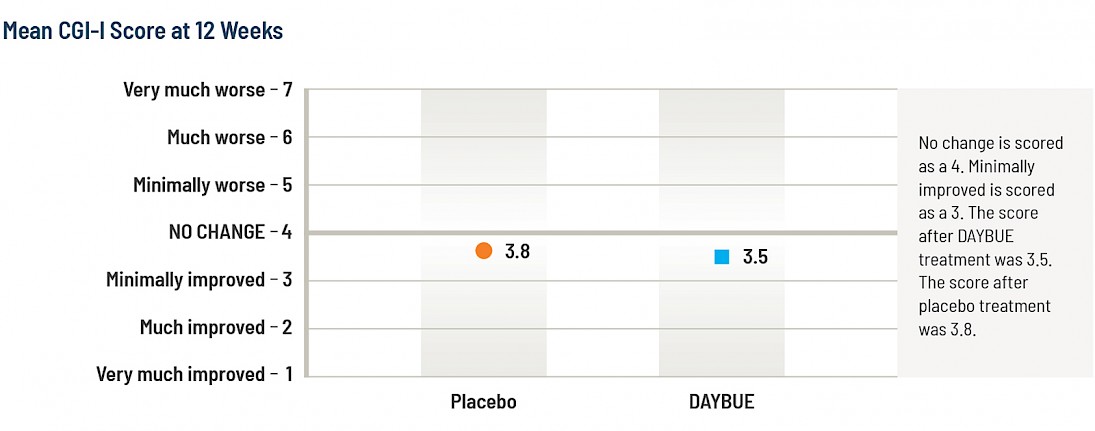
The average score (mean) of the CGI-I for the DAYBUE group at 12 weeks is 3.5, which is halfway between “no change” and “minimally improved,” while the placebo group average score was 3.8. The difference between the group of subjects that received DAYBUE and those that received placebo was statistically significant, in favor of DAYBUE.
Below is a graphical presentation of these results, showing the improvement scores for each group.
The graph below from the DAYBUE prescribing information reports the percentage of subjects rated in each specific CGI-I category.
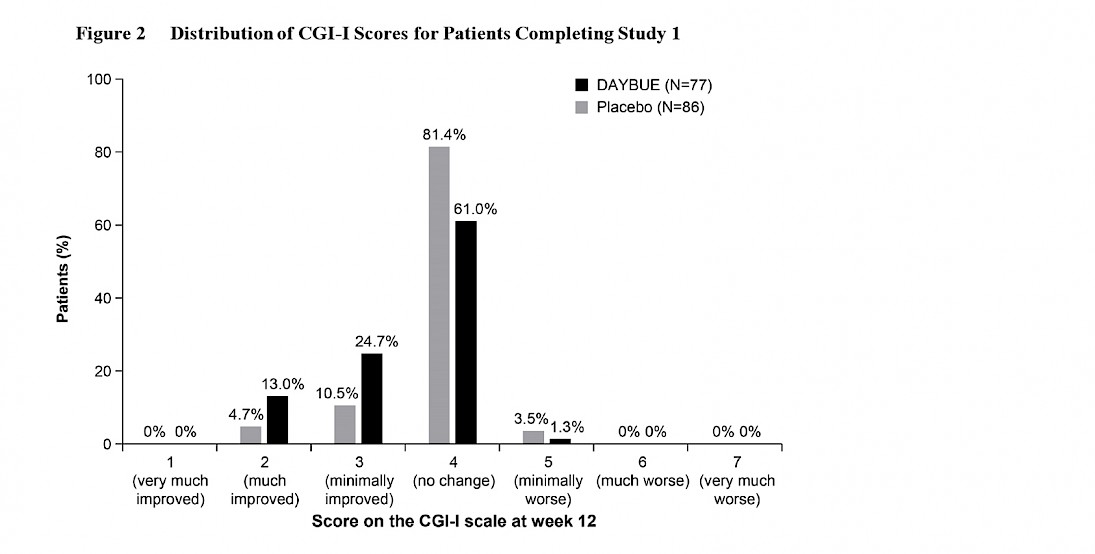
The majority of patients, 61%, were rated as “no change” on DAYBUE. Historically, scores of 1 or 2 have been considered to represent clinically significant improvement. 13% of patients on DAYBUE and 4.7% of patients on placebo were rated to have clinically significant improvement.
These guidelines explain what the CGI-I scores mean.
Statistical Significance vs Clinical Significance
A difference that is statistically significant does not mean that the difference is clinically significant. A clinically significant improvement is enough improvement that an individual feels, functions, or survives better. Small statistically significant differences between groups can be clinically significant differences. Though DAYBUE achieved statistically significant separation from placebo, these data suggest that DAYBUE may provide a clinically significant reduction in symptoms in a limited subset (13%) of individuals with Rett syndrome.
What Symptoms Actually Improved?
The prescribing information states that DAYBUE is indicated for the treatment of Rett syndrome. No information is provided regarding the specific symptoms that were considered improved in the LavenderTM study.
Side Effects
Diarrhea and weight loss are significant but potentially manageable side effects of DAYBUE. Diarrhea was the most common side effect, occurring in 85% of patients taking DAYBUE. Most were mild to moderate in severity. Half of patients had persistent diarrhea or recurrence even after dose interruptions, reductions, or adding anti-diarrhea medications.
In addition to the diarrhea, vomiting was reported in 29% of patients taking DAYBUE. 19% of patients receiving DAYBUE had side effects that led to withdrawal from the study, with the majority of withdrawals due to diarrhea.
Discontinuations
Both the RSBQ and CGI-I results reported above are based on the patients who completed the trial. 187 patients started the LavenderTM trial and 24 patients withdrew during the 12 weeks of treatment before completing the trial. 153 patients continued in the open-label LILAC-1 study, where everyone received DAYBUE for up to 40 weeks. As stated in Acadia’s community letter 46% of patients withdrew from the LILAC-1 trial before the end of the 40-week study.
Pricing
Acadia announced on their webcast that the list price of DAYBUE is $21.10 per mL. That comes to $9,495 per 450 mL bottle. Given the dosage included in the prescribing information, cost per patient, depending on weight, will be between $385,075 and $924,180 per year. Acadia has not yet announced if there will be discounts or rebates on pricing.
Food and Drug Administration (FDA)
The FDA approves medications when the potential benefit to patients outweighs the risks. FDA does not set or contribute to pricing of medications. FDA approval allows medications to be marketed in the United States only. Separate regulatory approvals are required for medications to be approved and marketed in other countries.
Access to DAYBUE Outside the USA
Acadia Pharmaceuticals holds the rights to market DAYBUE for Rett syndrome and other indications in North America. As per Acadia they plan to seek approval in Canada but have not announced plans for Mexico.
Neuren Pharmaceuticals retains the rights to market outside of North America. To our knowledge, Neuren has not announced if it will seek approval to market the drug outside of North America.
Key Summary Facts of LavenderTM and LILAC-1 Trials
- The majority of patients, 61%, taking DAYBUE were rated as “no change” on the CGI-I in the LavenderTM study.
- 13% of patients were rated as “much improved” on the CGI-I in the LavenderTM study.
- No data are provided regarding which specific symptoms get better in the LavenderTM study.
- 85% of patients treated with DAYBUE had diarrhea and 29% had vomiting. The prescribing information contains recommendations on managing the diarrhea.
- In the open label extension trial, LILAC-1, 46% of patients discontinued use of DAYBUE before the end of the study.
In Conclusion
The FDA approval of a drug for Rett syndrome is welcome as it raises the profile of Rett syndrome in the pharmaceutical world and shows there is a regulatory path for Rett. We expect this added visibility will incentivize other companies to explore adding Rett to their pipelines.
It’s important that every family considering DAYBUE as a treatment for their loved one make their own risk: benefit assessment with their physician. DAYBUE is not a cure. The data from the trial show that there will likely be no change for the majority of patients. A subset of patients may improve. The specifics on what symptoms improve have not been disclosed.


